| The atoms and molecules that make up any piece of matter are in constant random motion. This motion represents a form of energy known as thermal energy—or, more commonly, heat. The quantity we call temperature is a direct measure of this internal motion: the higher an object's temperature, the faster, on average, the random motion of its constituent particles. More precisely, the temperature of a piece of matter specifies the average thermal energy of the particles it contains.
Our familiar Fahrenheit temperature scale, like the archaic English system in which length is measured in feet and weight in pounds, is of somewhat dubious value. In fact, the "degree Fahrenheit" is now a peculiarity of American society. Most of the world uses the Celsius scale of temperature measurement (also called the centigrade scale). In the Celsius system, water freezes at 0 degrees (0° C) and boils at 100 degrees (100° C), as illustrated in the accompanying figure.
There are, of course, temperatures below the freezing point of water. Although we know of no matter anywhere in the universe that is actually |
this cold, temperatures can in theory reach as low as -273.15° C. This is the temperature at which atomic and molecular motion all but ceases. It is convenient to construct a temperature scale based on this lowest possible temperature, or absolute zero. Scientists commonly use such a scale, called the Kelvin scale in honor of the nineteenth-century British physicist Lord Kelvin. Since it takes absolute zero as its starting point, the Kelvin scale differs from the Celsius scale by 273.15°. In this book, we round off the decimal places and simply use
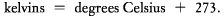
Thus,
- Translational motion ceases at 0 kelvins (0 K).
- Water freezes at 273 kelvins (273 K).
- Water boils at 373 kelvins (373 K).
Note that the unit is "kelvins," or "K," not "degrees kelvin" or "° K." (Occasionally, the term "degrees absolute" is used instead.) |