Figure 28.1 (see also the chapter-opening art) identifies seven major phases in the history of the universe: particulate, galactic, stellar, planetary, chemical, biological, and cultural evolution. Together, these evolutionary stages make up the grand sweep of cosmic evolution—the continuous transformation of matter and energy that has led to the appearance of life and civilization on Earth. The first four phases represent, in reverse order, the contents of this book. We now expand our field of view beyond astronomy to include the other three.
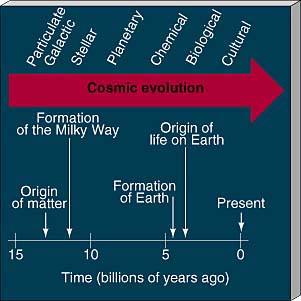
Figure 28.1 Some highlights of cosmic history are noted along this arrow of time, from the beginning of the universe to the present. Noted along the top of the arrow are the major phases of cosmic evolution.
From the Big Bang, to the formation of galaxies, to the birth of the solar system, to the emergence of life, to the evolution of intelligence and culture, the universe has evolved from simplicity to complexity. We are the result of an incredibly complex chain of events that spanned billions of years. Were those events random, making us unique, or are they in some sense natural, so that technological civilization is inevitable? Put another way, are we alone in the universe, or are we just one among countless other intelligent life-forms in our Galaxy? In this chapter we consider the development of life on Earth and try to assess the likelihood of finding intelligent life elsewhere in the cosmos.
Before embarking on our study, we need a working definition of life. This seemingly simple task is not an easy one—the distinction between the living and the nonliving is not as obvious as we might at first think. Though most physicists would agree on the definitions of matter and energy, biologists have not arrived at a clear-cut definition of life. Generally speaking, scientists regard the following as characteristics of living organisms: (1) they can react to their environment and can often heal themselves when damaged; (2) they can grow by taking in nourishment from their surroundings and processing it into energy; (3) they can reproduce, passing along some of their own characteristics to their offspring; and (4) they have the capacity for genetic change and can therefore evolve from generation to generation so as to adapt to a changing environment.
These rules are not strict, and there is great leeway in interpreting them. Stars, for example, react to the gravity of their neighbors, grow by accretion, generate energy, and "reproduce" by triggering the formation of new stars, but no one would suggest that they are alive. A virus (see Interlude 28-1) is crystalline and inert when isolated from living organisms, but once inside a living system, it exhibits all the properties of life, seizing control of a living cell and using the cell's own genetic machinery to grow and reproduce. Most researchers now believe that the distinction between living and nonliving is more one of structure and complexity than a simple checklist of rules.
The general case in favor of extraterrestrial life is summed up in what are sometimes called the assumptions of mediocrity: (1) because life on Earth depends on just a few basic molecules, and (2) because the elements that make up these molecules are (to a greater or lesser extent) common to all stars, and (3) if the laws of science we know apply to the entire universe, as we have supposed throughout this book, then—given sufficient time—life must have originated elsewhere in the cosmos. The opposing view maintains that intelligent life on Earth is the product of a series of extremely fortunate accidents—astronomical, geological, chemical, and biological events unlikely to have occurred anywhere else in the universe. The purpose of this chapter is to examine some of the arguments for and against these viewpoints.
What information do we have about the earliest stages of planet Earth? Unfortunately, not very much. Geological hints about the first billion years or so were largely erased by violent surface activity as volcanoes erupted and meteorites bombarded our planet; subsequent erosion by wind and water has seen to it that little evidence has survived to the present. Scientists believe that the early Earth was barren, with shallow, lifeless seas washing upon grassless, treeless continents. Outgassing from our planet's interior through volcanoes, fissures, and geysers produced an atmosphere rich in hydrogen, nitrogen, and carbon compounds and poor in free oxygen. As Earth cooled, ammonia, methane, carbon dioxide, and water formed. The stage was set for the appearance of life.
The surface of the young Earth was a very violent place. Natural radioactivity, lightning, volcanism, solar ultraviolet radiation, and meteoritic impacts all provided large amounts of energy that eventually shaped the ammonia, methane, carbon dioxide, and water into more complex molecules known as amino acids and nucleotide bases—organic (carbon-based) molecules that are the building blocks of life as we know it. Amino acids build proteins, and proteins control metabolism, the daily utilization of food and energy by means of which organisms stay alive and carry out their vital activities. Sequences of nucleotide bases form genes—parts of the DNA molecule—which direct the synthesis of proteins and thus determine the characteristics of the organism. These same genes also carry the organism's hereditary characteristics from one generation to the next in reproduction. In all living creatures on Earth—from bacteria to amoebas to humans—genes mastermind life, and proteins maintain it.
The idea that complex molecules could have evolved naturally from simpler ingredients found on the primitive Earth has been around since the 1920s. The first experimental verification was provided in 1953 when scientists Harold Urey and Stanley Miller, using laboratory equipment somewhat similar to that shown in Figure 28.2, took a mixture of the materials thought to be present on Earth long ago—a "primordial soup" of water, methane, carbon dioxide, and ammonia—and energized it by passing an electrical discharge ("lightning") through the gas. After a few days they analyzed their mixture and found that it contained many of the same amino acids found today in all living things on Earth. About a decade later, scientists succeeded in constructing nucleotide bases in a similar manner. These experiments have been repeated in many different forms, with more realistic mixtures of gases and a variety of energy sources, but always with the same basic outcomes.
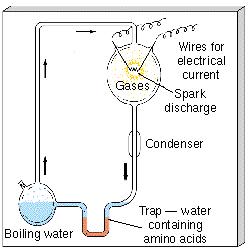
Figure 28.2 This chemical apparatus is designed to synthesize complex biochemical molecules by energizing a mixture of simple chemicals. A mixture of gases (ammonia, methane, carbon dioxide, water vapor) is placed in the upper bulb to simulate the primordial Earth atmosphere and then energized by spark-discharge electrodes. After about a week, amino acids and other complex molecules are found in the trap at the bottom, which simulates the primordial oceans into which heavy molecules produced in the overlying atmosphere would have fallen.
Although none of these experiments has ever produced a living organism, or even a single strand of DNA, they do demonstrate conclusively that "biological" molecules can be synthesized by strictly nonbiological means, using raw materials available on the early Earth. More advanced experiments, in which amino acids are united under the influence of heat, have fashioned proteinlike blobs that behave to some extent like true biological cells. Such near-protein material resists dissolution in water (so it would remain intact when it fell from the primitive atmosphere into the ocean) and tends to cluster into small droplets called microspheres—a little like oil globules floating on the surface of water. Figure 28.3 shows some of these proteinlike microspheres. The walls of these laboratory-made droplets permit the inward passage of small molecules, which then combine within the droplet to construct more complex molecules too large to pass back out through the walls. As the droplets "grow," they tend to "reproduce," forming smaller droplets.

Figure 28.3 These carbon-rich, proteinlike droplets display the clustering of as many as a billion amino acid molecules in a liquid. Droplets can "grow," and parts of droplets can separate from the "parent" to become new individual droplets (as at A, B, C). The scale of 2.5 microns noted here is 1/4000 of a centimeter.
Can we consider these proteinlike microspheres to be alive? Almost certainly not. Most biochemists would say that the microspheres are not life itself, but they contain many of the basic ingredients needed to form life. The microspheres lack the hereditary molecule DNA. However, as illustrated in Figure 28.4, they do have similarities to ancient cells found in the fossil record. Thus, while no actual living cells have yet been created "from scratch" in any laboratory, many biochemists feel that the chain of events leading from simple nonbiological molecules almost to the point of life itself has been amply demonstrated.
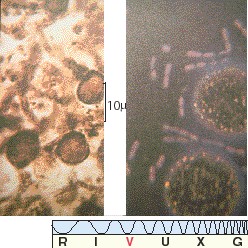
Figure 28.4 The photograph on the left, taken through a microscope, shows a fossilized organism found in sediments radioactively dated as 2 billion years old. This primitive system possesses concentric spheres or walls connected by smaller spheroids. The roundish fossils here measure about a thousandth of a centimeter. The photograph on the right, also taken through a microscope and on approximately the same scale, displays modern blue-green algae.
Recently, however, a dissenting view has emerged. Some scientists have argued that Earth's primitive atmosphere might not in fact have been a particularly suitable environment for the production of complex molecules. Instead, they say, there may not have been sufficient energy available to power the chemical reactions, and the early atmosphere may not have contained enough raw material for the reactions to have become important in any case. These researchers suggest that much, if not all, of the organic material that combined to form the first living cells was produced in interstellar space and subsequently arrived on Earth in the form of interplanetary dust and meteors that did not burn up during their descent through the atmosphere.
Interstellar molecular clouds are known to contain very complex molecules, and large amounts of organic material were detected on comet Halley by space probes when Halley last visited the inner solar system (Section 14.2) Similarly complex molecules were observed on comet Hale—Bopp. (Interlude 14-2)) Thus, the idea that organic matter is constantly raining down on Earth from space in the form of interplanetary debris is quite plausible. Whether or not this was the primary means by which complex molecules first appeared in Earth's oceans remains unclear. For now, the issue is unresolved.
However the basic materials appeared on Earth, we know that life did appear. The fossil record chronicles how life on Earth became widespread and diversified over the course of time. The study of fossil remains shows the initial appearance about 3.5 billion years ago of simple one-celled organisms such as blue-green algae. These were followed about 2 billion years ago by more complex one-celled creatures, like the amoeba. Multicellular organisms such as sponges did not appear until about 1 billion years ago, after which there flourished a wide variety of increasingly complex organisms—insects, reptiles, mammals, and humans.
The fossil record leaves no doubt that biological organisms have changed over time—all scientists accept the reality of biological evolution. As conditions on Earth shifted and Earth's surface evolved, those organisms that could best take advantage of their new surroundings succeeded and thrived—often at the expense of those organisms that could not make the necessary adjustments and consequently became extinct. What led to these changes? Chance. An organism that happened to have a certain useful genetically determined trait—for example, the ability to run faster, climb higher, or even hide more easily—would find itself with the upper hand in a particular environment. This organism was therefore more likely to reproduce successfully, and its advantageous characteristic would then be more likely to be passed on to the next generation. The evolution of the rich variety of life on our planet, including human beings, occurred as chance mutations—changes in genetic structure—led to changes in organisms over millions of years.
What about the development of intelligence? Many anthropologists believe that, like any other highly advantageous trait, intelligence is strongly favored by natural selection. As humans learned about fire, tools, and agriculture, the brain became more and more elaborate. The social cooperation that went with coordinated hunting efforts was another important competitive advantage that developed as brain size increased. Perhaps most important of all was the development of language. Indeed, some anthropologists have gone so far as to suggest that human intelligence is human language. By communicating, individuals could signal one another while hunting food or seeking protection. Now our ancestors could share ideas as well as food and shelter. Experience, stored in the brain as memory, could be passed down from generation to generation. A new kind of evolution had begun, namely, cultural evolution, the changes in the ideas and behavior of society. Our more recent ancestors have created, within only the past 10,000 years or so, the entirety of human civilization.
To put all this into historical perspective, let's imagine the entire lifetime of Earth to be 46 years rather than 4.6 billion years. We have no reliable record of the first decade of our planet's existence. Life originated at least 35 years ago, when Earth was about 10 years old. Our planet's middle age is largely a mystery, although we can be sure that life continued to evolve and that generations of mountain chains and oceanic trenches came and went. Not until about 6 years ago did abundant life flourish throughout Earth's oceans. Life came ashore about 4 years ago, and plants and animals mastered the land only about 2 years ago. Dinosaurs reached their peak about 1 year ago, only to die suddenly about 4 months later. (Interlude 14-2)) Humanlike apes changed into apelike humans only last week, and the latest ice ages occurred only a few days ago. Homo sapiens—our species—did not emerge until about 4 hours ago. Agriculture was invented within the last hour, and the Renaissance—along with all of modern science—is just 3 minutes old!