Simple one-celled life-forms reigned supreme on Earth for most of our planet's history. It took time—a great deal of time—for life to emerge from the oceans, to evolve into simple plants, to continue to evolve into complex animals, and to develop intelligence, culture, and technology. Have those (or similar) events occurred elsewhere in the universe? Let's try to assess what little evidence we have on the subject.
"Life as we know it" is generally taken to mean carbon-based life that originated in a liquid water environment—in other words, life on Earth. Is there any reason to suppose that such life might exist elsewhere in our solar system? The answer appears to be no. It seems that no environment in the solar system besides Earth is particularly well suited for sustaining Earthlike life.
The Moon and Mercury lack liquid water, protective atmospheres, and magnetic fields, and so these two bodies are subjected to fierce bombardment by solar ultraviolet radiation, the solar wind, meteoroids, and cosmic rays. Simple molecules could not possibly survive in such hostile environments. Venus has far too much protective atmosphere! Its dense, dry, scorchingly hot atmospheric blanket effectively rules it out as a possible abode for life.
The jovian planets have no solid surfaces (although some researchers maintain that life could have evolved in their atmospheres), and Pluto and most of the moons of the outer planets are too cold. However, the possibility of liquid water below Europa's icy surface has refueled speculation about the development of life there. (Sec. 11.5) Saturn's moon Titan, with its atmosphere of methane, ammonia, and nitrogen and possibly with some liquid on its surface, is conceivably a site where life might have arisen, although the results of the 1980 Voyager 1 flyby suggest that Titan's frigid surface conditions are inhospitable to anything familiar to us.
(Sec. 12.5)
What about the cometary and meteoritic debris that orbits within our solar system? Comets contain many of the basic ingredients for life—for instance, ammonia, methane, and water vapor—and although comets are frozen, their icy matter warms while nearing the Sun. Indeed, some heavy molecules have been observed in comet spectra. In addition, a small fraction of the meteorites that survive the plunge to Earth's surface do contain organic compounds. The Murchison meteorite (Figure 28.5), which fell near Murchison, Australia, in 1969, is a well-studied example. Located soon after crashing to the ground, this meteorite contains many of the well-known amino acids normally found in living cells. The moderately large molecules found in meteorites and in interstellar clouds are our only evidence that chemical evolution has occurred elsewhere in the universe. Most researchers regard this organic matter as prebiotic—that is, matter that could eventually lead to life but that has not yet done so.
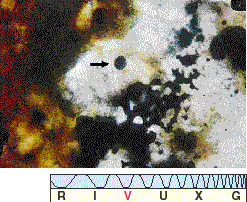
Figure 28.5 The Murchison meteorite contains relatively large amounts of amino acids and other organic material, indicating that chemical evolution of some sort has occurred beyond our own planet. In this magnified view of a meteorite fragment, the arrow points to a microscopic sphere of organic matter.
The planet most likely to harbor life (or to have harbored it in the past) seems to be Mars. This planet seems harsh by Earth standards—liquid water is scarce, the atmosphere is thin, and the lack of magnetism and an ozone layer allows the solar high-energy particles and ultraviolet radiation to reach the surface unabated. But the Martian atmosphere was thicker, and the surface warmer and much wetter in the past.
(Secs. 10.4, 10.5) In the hope that life might once have evolved on Mars as it did on Earth, the Viking lander carried a television camera to seek fossilized remnants of large plants or animals. No fossils of any kind were seen. Mars Pathfinder also surveyed part of the Martian surface, again without finding any evidence of present or past life on Mars.
(Sec. 10.4) The Viking landers scooped up Martian soil (Figure 28.6) and tested for life by conducting chemical experiments designed to detect the waste gases and other products of metabolic activity, but no unambiguous evidence of Martian life has emerged.
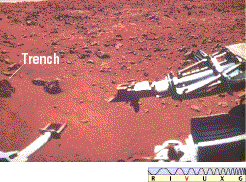
Figure 28.6 A trench dug by the "arm" of one of the Viking robots can be seen at the left of this photograph. Soil samples were scooped up and taken inside the robot, where instruments tested them for chemical composition and any signs of life.
The consensus among biologists and chemists today is that Mars does not house any life similar to that on Earth. However, some scientists think that a different type of biology may be operating on the Martian surface. They suggest that Martian microbes capable of eating and digesting oxygen-rich compounds in the Martian soil could also explain the Viking results. This speculation will be greatly strengthened if recent announcements of fossilized bacteria in meteorites originating on Mars are confirmed. Interlude 10-2 In addition, microbial life may reside in more habitable regions on Mars, such as near the moist polar caps. It seems that a solid verdict regarding life on Mars will not be reached until we have thoroughly explored our intriguing neighbor.
It thus appears that no environment in the solar system besides Earth is particularly well suited for sustaining life—at least life similar to that on Earth. However, some scientists have suggested that different types of biology may be at work out there, ones that we cannot recognize and that we do not know how to test for. What other biologies might exist?
Some scientists have pointed out that the abundant element silicon has chemical properties somewhat similar to those of carbon and have suggested it as a possible alternative to carbon as the basis for living organisms. Ammonia (made of the common elements hydrogen and nitrogen) is sometimes put forward as a possible liquid medium in which life might develop, at least on a planet cold enough for ammonia to exist in the liquid state. Together or separately, these alternatives would surely give rise to organisms with radically different biochemistries from those we know on Earth. Conceivably, we might have difficulty even recognizing these organisms as alive.
Although the possibility of such alien life forms is a fascinating scientific problem, most biologists would argue that chemistry based on carbon and water is the one most likely to give rise to life. Carbon's flexible chemistry and water's wide liquid temperature range are just what are needed for life to develop and thrive. Silicon and ammonia seem unlikely to fare as well as the bases for advanced life-forms. Silicon's chemical bonds are weaker than those of carbon and may not be able to form complex molecules—an apparently essential aspect of carbon-based life. Also, the colder the environment, the less energy there is to drive biological processes. The low temperatures necessary for ammonia to be liquid might inhibit or even prevent completely the chemical reactions leading to the equivalent of amino acids and nucleotide bases.
Still, we must admit that we know next to nothing about noncarbon, nonwater biochemistries, for the very good reason that there are no examples of them to study experimentally. We can speculate about alien life-forms and try to make general statements about their properties, but we can say little of substance about them.