We now have all the ingredients needed to complete our story of the creation of the elements, begun in Chapter 21 but never quite finished.
(Sec. 21.4) The theory of stellar nucleosynthesis accounts very well for the observed abundances of heavy elements in the universe, but there are discrepancies between theory and observations when it comes to the abundances of the light elements, especially helium. Simply put, there is far more helium in the universe— about 25 percent by mass—than can be explained by nuclear fusion in stars. No matter where they look, and no matter how low a star's abundance of heavy elements may be, astronomers find that there is a minimum amount of helium in all stars. The accepted explanation is that this base level of helium is primordial—that is, it was created during the early, hot epochs of the universe, before any stars had formed. The production of elements heavier than hydrogen by nuclear fusion shortly after the Big Bang is called primordial nucleosynthesis.
By about 100 s after the Big Bang, the temperature had fallen to about 1 billion K, and "apart from exotic" dark-matter particles, matter in the universe consisted of electrons, protons, and neutrons, with the protons outnumbering the neutrons by about 5 to 1. The stage was set for nuclear fusion to occur. Protons and neutrons combined to produce deuterium nuclei:
Although this reaction must have occurred very frequently during the lepton epoch, the temperature then was still so high that the deuterium nuclei were broken apart by high-energy gamma rays as soon as they formed. The universe had to wait until it became cool enough for the deuterium to survive. This waiting period is sometimes called the deuterium bottleneck.
Only when the temperature of the universe fell below about 900 million K, roughly 2 minutes after the Big Bang, was deuterium at last able to form and endure. Once that occurred, the deuterium was quickly converted into heavier elements by numerous reactions:
along with many others. The result was that once the universe passed the deuterium bottleneck, fusion proceeded rapidly and large amounts of helium were formed. In just a few minutes most of the free neutrons were consumed, leaving a universe whose matter content was primarily hydrogen and helium. Figure 27.5 illustrates some of the reactions responsible for helium formation. Contrast it with Figure 16.28, which depicts how helium is formed today in the cores of main-sequence stars like the Sun. (The proton—proton chain that powers the Sun played no significant role in primordial helium formation. The proton—proton reaction that starts the chain is very slow compared with the proton—neutron reaction discussed here and is important in the Sun only because the solar interior contains no free neutrons to make the latter reaction possible.) (Sec. 16.5)
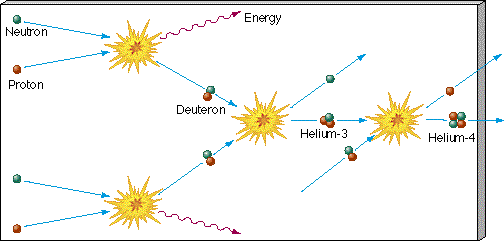
Figure 27.5 Some of the reaction sequences that led to the formation of helium in the early universe. Compare this figure with Figure 16.26, which depicts the proton—proton chain in the Sun.
We might imagine that fusion could have continued to create heavier and heavier elements, just as in the cores of stars, but this did not occur. In stars, the density and the temperature both increase slowly with time, allowing more and more massive nuclei to form, but in the early universe exactly the opposite was true. The temperature and density were both decreasing rapidly, making conditions less and less favorable for fusion as time went on. Even before the supply of neutrons was completely used up, the nuclear reactions had effectively ceased. Reactions between helium nuclei and protons may also have formed trace amounts of lithium (the next element beyond helium) by this time, but for all practical purposes, the expansion of the universe caused fusion to stop at helium. The brief epoch of primordial nucleosynthesis was over about 15 minutes after it began.
By the end of the nuclear epoch, some 1000 s after the Big Bang, the temperature of the universe was about 300 million K and the cosmic elemental abundance was set. Careful calculations indicate that about 1 helium nucleus had formed for every 12 protons remaining. Because a helium nucleus is four times more massive than a proton, helium accounted for about one quarter of the total mass of matter in the universe:
The remaining 75 percent of the matter in the universe was hydrogen. It would be almost a billion years before nucleosynthesis in stars would change these numbers.
The foregoing calculation implies that all cosmic objects should contain at least 25 percent helium by mass. The figure for the Sun, for example, is about 28 percent. However, it is difficult to disentangle the contributions to the present-day helium abundance from primordial nucleosynthesis and later hydrogen burning in stars. Our best hope of determining the amount of primordial helium is to study the oldest stars known, since they formed early on, before stellar nucleosynthesis had had time to change significantly the helium content of the universe. Unfortunately, stars surviving from that early time are of low mass and hence quite cool, making the helium lines in their spectra very weak and hard to measure accurately.
(Secs. 17.6, 17.9) Nevertheless, despite this uncertainty, the observations are generally consistent with the theory just described.
Bear in mind that while all this was going on matter was just an insignificant "contaminant" in the radiation-dominated universe. Radiation outmassed matter by about a factor of 5000 at the time helium formed. The existence of helium is very important in determining the structure and appearance of stars today, but its creation was completely irrelevant to the evolution of the universe at the time.
During the nuclear epoch, although most deuterium was quickly fused into helium as soon as it formed, a small amount was left over when the primordial nuclear reactions ceased. Observations of deuterium—especially those made by orbiting satellites able to capture deuterium's strongest spectral feature, which happens to be emitted in the ultraviolet part of the spectrum—indicate a present-day abundance of about 2 deuterium nuclei for every 100,000 protons. However, unlike helium, deuterium is not produced to any significant degree in stars (in fact, deuterium tends to be destroyed in stars), so any deuterium we see today must be primordial.
This observation is of great importance to astronomers because it provides them with a sensitive method—and one that is completely independent of the techniques discussed in previous chapters—of probing the present-day density of matter in the universe. According to theory, as illustrated in Figure 27.6, the denser the universe is today, the more particles there were at early times to react with deuterium as it formed, and the less deuterium was left over when nucleosynthesis ended. Comparison of the observed deuterium abundance (marked on the figure) with the theoretical results implies a present-day density of at most 3 10-28 kg/m3—only a few percent of the critical density. Thus, measurements of the cosmic deuterium abundance provide us with a remarkably firm estimate of
0.03.

Figure 27.6 The present-day abundance of deuterium depends strongly on the amount of matter present at early times, and this, in turn, determines the present-day density of the universe. Thus, measuring the amount of deuterium in the universe gives us an estimate of the overall density of matter. The best deuterium measurements are marked; they imply that the density of matter in the universe is at most a few percent of the critical value.
But before we jump to the conclusion that the universe is open and will expand forever, we must make one very important qualification. Primordial nucleosynthesis as just described depends only on the presence of protons and neutrons in the early universe. Thus, measurements of the abundance of helium and deuterium tell us only about the density of "normal" matter—matter made up of protons and neutrons—in the cosmos. This finding has a momentous implication for the overall composition of the universe. As we saw earlier, astronomers have concluded, on the basis of studies of the motions of galaxies in clusters and superclusters, that 0 is at least 0.2 or 0.3, and may possibly be much more.
(Sec. 26.4) If this reasoning is correct, and if the density of normal matter is only a few percent of the critical value, then we are forced to admit that not only is most of the matter in the universe dark, but most of the dark matter is not composed of protons and neutrons.
Thus, the bulk of the matter in the universe apparently exists in the form of elusive subatomic particles (discussed as dark-matter candidates in Chapter 23), whose nature we do not fully understand and whose very existence has yet to be conclusively demonstrated in laboratory experiments. (Sec. 23.6) For the sake of brevity we will adopt from here on the convention that the term "dark matter" refers only to these unknown particles and not to "stellar" dark matter, such as black holes and white dwarfs (also discussed in Chapter 23), which are made of normal matter.
A few thousand years after the Big Bang, radiation ceased to be the dominant component of the universe. The Matter Era had begun. At the start of the atomic epoch, matter consisted of electrons, protons, helium nuclei (formed by primordial nucleosynthesis), and dark matter. The temperature was several tens of thousands of kelvins—far too hot for atoms of hydrogen to exist, although some helium ions may already have formed. During the next few hundred thousand years, a major change occurred. The universe expanded by another factor of 10, the temperature dropped to a few thousand kelvins, and electrons and nuclei combined to form neutral atoms. By the time the temperature had fallen to 4500 K, the universe consisted of atoms, photons, and dark matter.
The period during which nuclei and electrons combined to form atoms is often called the epoch of decoupling, for it was during this period that the radiation background parted company with normal matter. At early times, when matter was ionized, the universe was filled with large numbers of free electrons, which interacted frequently with electromagnetic radiation of all wavelengths. As a result, a photon could not travel far before encountering an electron and scattering off it. In effect, the universe was opaque to radiation (rather like the deep interior of a star like the Sun). Matter and radiation were strongly "tied," or coupled, to one another by these interactions.
When the electrons combined with nuclei to form atoms of hydrogen and helium, however, only certain wavelengths of radiation—the ones corresponding to the spectral lines of those atoms—could interact with matter. (Sec. 4.2) Radiation of other wavelengths could travel virtually forever without being absorbed. The universe became nearly transparent. From that time on, photons passed generally unhindered through space. As the universe expanded, the radiation simply cooled, eventually to become the microwave background we see today.
The microwave photons now detected on Earth have been traveling through the universe ever since they decoupled. Their last interaction with matter (at the epoch of decoupling) occurred when the universe was a few hundred thousand years old and roughly 1500 times smaller (and hotter) than it is today—that is, at a redshift of 1500. As illustrated in Figure 27.7, the epoch of atom formation created a kind of "photosphere" in the universe, completely surrounding Earth at a distance of approximately 9000 Mpc, the distance at which the photons last interacted before they decoupled. (More Precisely 25-1 ) On our side of the photosphere—that is, since decoupling—the universe is transparent. On the far side—before decoupling—it was opaque. Thus, by observing the microwave background, we are probing conditions in the universe almost all the way back in time to the Big Bang, in much the same way as studying sunlight tells us about the surface layers of the Sun.
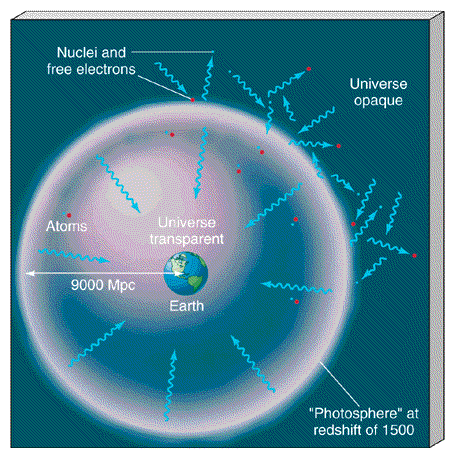
Figure 27.7 When atoms formed, the universe became virtually transparent to radiation. Thus, observations of the cosmic background radiation allow us to study conditions in the universe around a time at a redshift of 1500, when the temperature dropped below about 4500 K. For an explanation of how we can see a region of space 9000 Mpc (30 billion light years) away when the universe is just 10 billion years old, see More Precisely 25-1.